
SPRAVATO® (esketamine) is a groundbreaking nasal spray approved by the FDA for adults struggling with two challenging types of depression:

Treatment-Resistant Depression (TRD): Designed for individuals who have not responded to at least two different antidepressants.

Major Depressive Disorder (MDD) with Acute Suicidal Ideation or Behavior: A rapid-acting option for individuals experiencing intense symptoms and an urgent need for stabilization.
Unlike traditional oral antidepressants that may take weeks to work, SPRAVATO® has been shown to deliver symptom relief in as little as 24 hours.



Start with a phone consultation. If eligible, you'll meet with our mental health professionals for a full evaluation and medical history review. We’ll also verify insurance coverage to avoid surprises.

After approval, your SPRAVATO® sessions will be scheduled at our clinic. You’ll self-administer the spray in a calm, comfortable environment while monitored by our staff for at least two hours.
Treatment begins with an induction phase (typically two sessions weekly) and transitions to maintenance dosing. We customize frequency based on your individual progress and needs.

Our care team is with you every step of the way. We offer regular follow-ups, collaborate with your existing mental health providers, and continuously adapt your care plan.

Week 1-4
2 Treatments a Week

Week 5-8
1 Treatments a Week

Week 9+
1 Treatments a Week Every 1-2 Weeks
Response Rate:
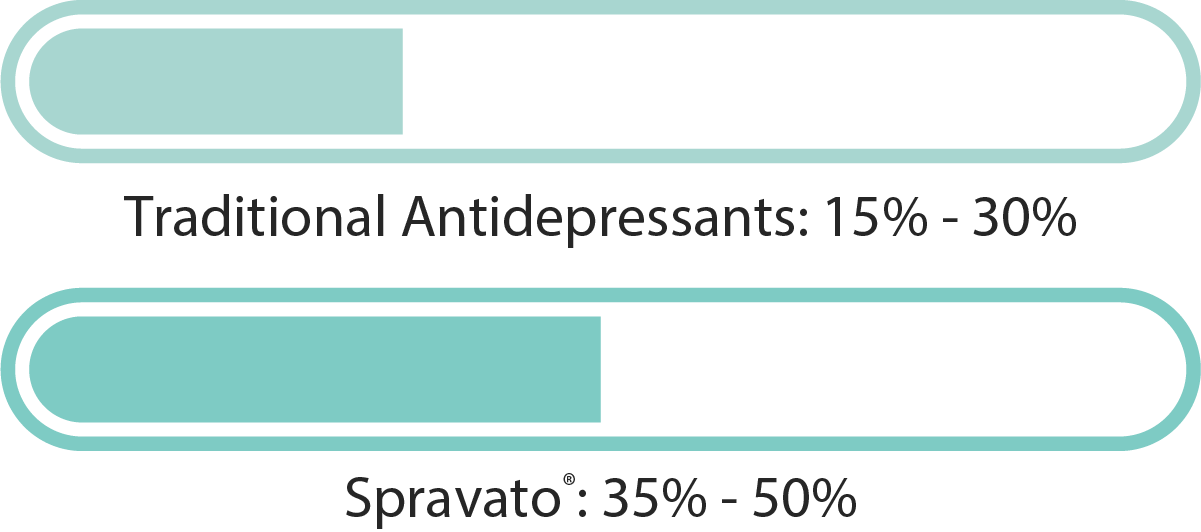
Conventional antidepressant medications generally yield positive treatment responses in about 40% to 60% of patients. In contrast, clinical research on SPRAVATO® (esketamine) has reported response rates reaching up to 70% (Popova et al., 2019, American Journal of Psychiatry).
Onset of Therapeutic Effect:
Whereas standard antidepressants may require between four to six weeks before users begin to notice any significant improvement, esketamine (SPRAVATO®) has been associated with symptom relief in as little as 24 hours, according to clinical trial data (BMC Psychiatry, 2022).
Sustained Remission Rates:
In a clinical trial, 43.8% of individuals who continued treatment with Spravato alongside an oral antidepressant at the three-month mark had entered remission from major depressive episodes.



We follow only FDA-approved treatment protocols.
Our clinic spaces are designed to support mental wellness.

We engage your existing care team to ensure consistent and holistic support.
We work with your insurance and handle all necessary paperwork.
Over 3,000+ Boise residents have used SPRAVATO® to elevate their lives.
No. SPRAVATO® must be administered in a clinical setting under supervision.
Download our informational materials to learn more about SPRAVATO® and how it may benefit your recovery journey.
Boise Ketamine Clinic is the premier ketamine treatment provider in Boise, ID. Our goal is to help you achieve a balance if life so you can start enjoying the things you love to do once again. We are a leading provider of medically supervised ketamine, ketamine assisted therapy: therapist guided, ketamine-assisted psychotherapy (KAP) for the treatment of mental health and chronic pain conditions. We are committed to providing our clients with the highest quality care in a safe and supportive environment.
© 2025 Boise Ketamine Clinic. All rights reserved.